amount of citric acid. The procedure commonly used to do this takes advantage of the known reactivity of citric acid with sodium hydroxide and is known as a titration. A titration is a means of quantitative analysis in which the substance to be measured (in a liquid solution) is reacted stoichiometrically with another reagent. Citric acid is a weak acid, and is thus best titrated using a strong base, such as sodium hydroxide, with the combination of a weak acid and a strong base producing an endpoint that corresponds to the range of phenolphthalein indicator (pH range of 8.3-10).

Chemical Equation For Baking Soda Citric Acid And Water Tessshebaylo

Merck 109435 Buffer solution (citric acid/sodium hydroxide/hydrogen chloride), traceable to SRM

MERCK 109435 pH 4 buffer solution
Buffer solution (citric acid/sodium hydroxide/hydrogenchloride), ph 4.0 traceble to SRM
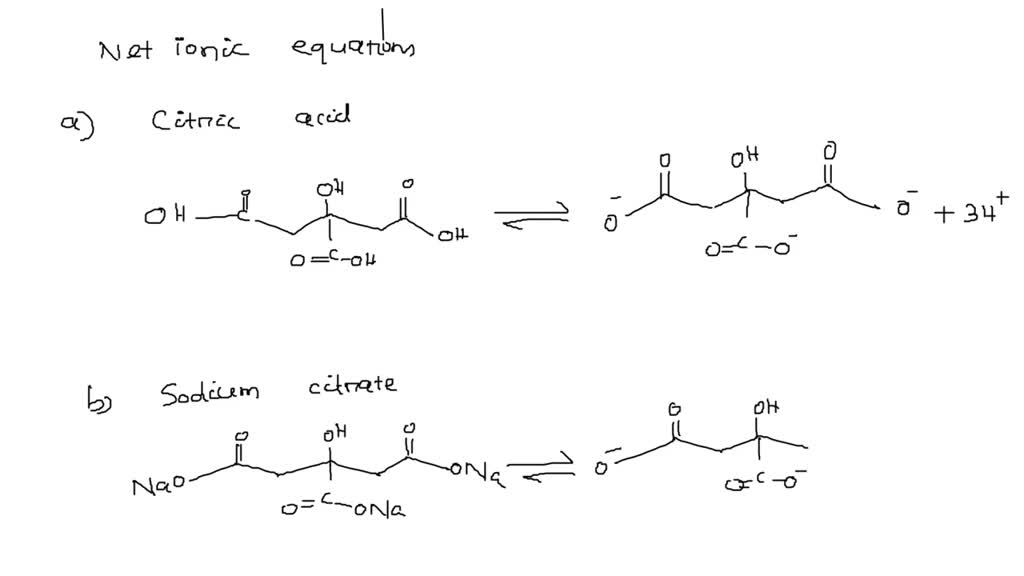
SOLVED Question 16 Look at the structure for citric acid (see Figure below) Various sodas

Buffer solution pH = 2,00 (20 °C) (Citric acid/Sodium hydroxide/Hydrochloric acid) 1L Southern
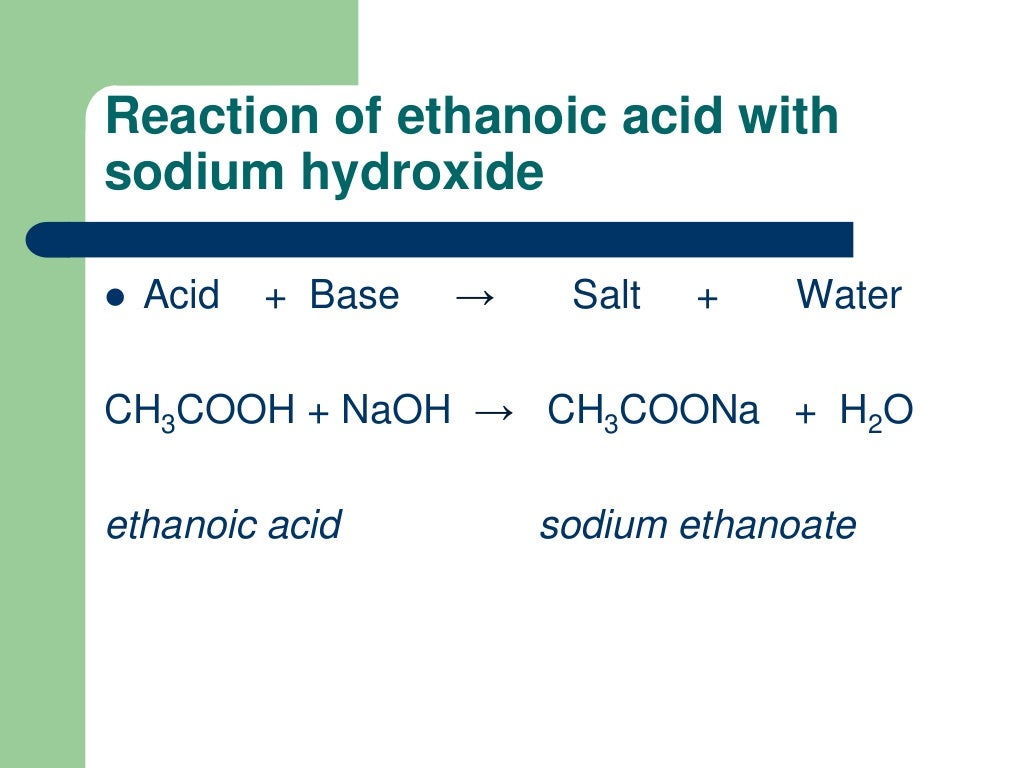
7.3 reactions as acids

MERCK 199016 (citric acid/sodium hydroxide), traceable to SRM from NIST and PTB pH 6.00 (25°C

How many h+(aq) can be generated by each citric acid molecule dissolved in water?

Citric Acid All Ingredients Plus

Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision
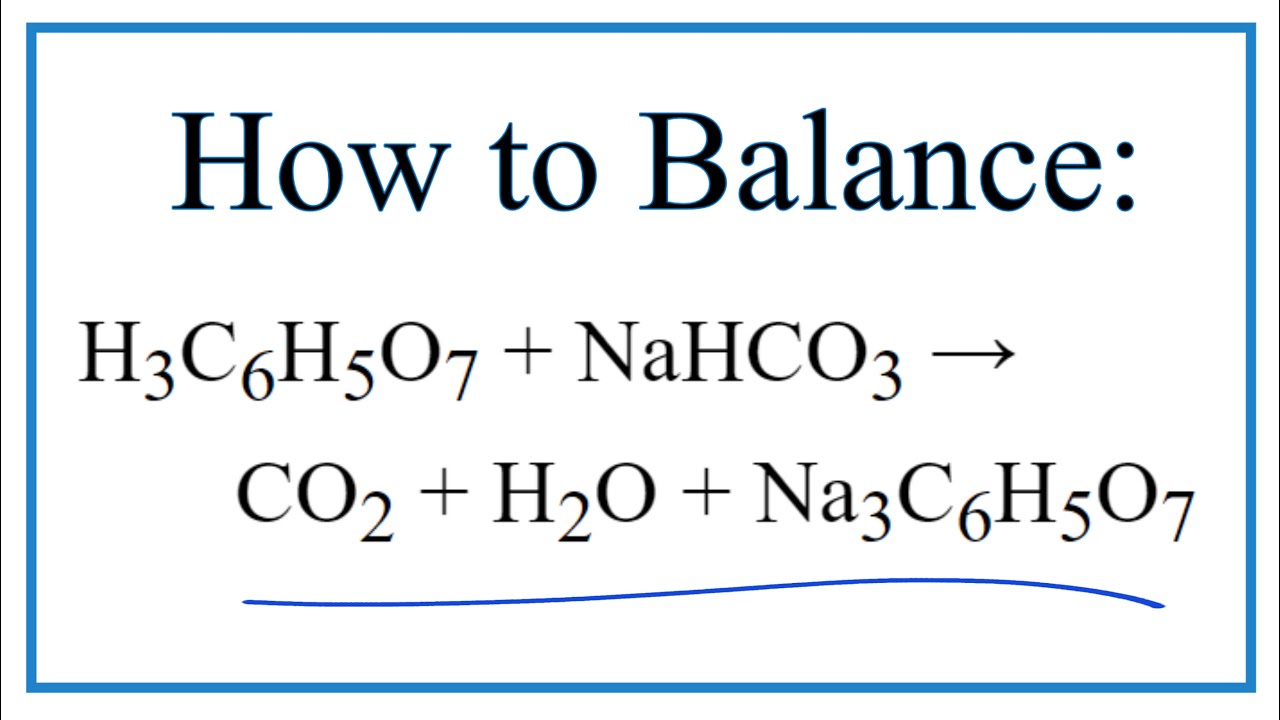
Citric Acid and Sodium Bicarbonate Reaction Equation

OneClass Please show ALL work. Citric acid and sodium hydroxide react with each other according
Citric Acid Monohydrate 500g + Sodium Bicarbonate 500g Set) CISOD Shopee Malaysia
[Solved] . 1. Citric acid appears as a white solid under room conditions.... Course Hero

Buffer solution (citric acid/sodium hydroxide/hydrogen chloride) tracable to SRM from NIST and

citric acid bulk citric acid anhydrous calcium hydroxide 95 food grade calcium hydroxide ca oh

Buffer solution (citric acid/sodium hydroxide/hydrogen chloride), traceable to SRM from NIST and

Buffer solution (citric acid/sodium hydroxide/hydrogen chloride) colour red traceable to SRM

Carboxylic acids with sodium hydroxide, sodium carbonate, sodium bicarbonate reaction YouTube
Prepare mL of distilled water in a suitable container. Add g of Citric Acid to the solution. Add g of Sodium Hydroxide to the solution. Add g of Hydrochloric Acid to the solution. Adjust solution to final desired pH using HCl or NaOH. Add distilled water until volume is L. Citrate-Sodium Citrate Buffer Calculator.. The third titration, C, has citric acid monohydrate as an unknown carboxylic acid to be determined by titration. A. Standardization of a sodium hydroxide solution. Citric acid can be used as a lower-cost alternative reagent to potassium hydrogen phthalate 1 (KHP) for standardizing 0.1 M NaOH solutions. High-purity solid citric acid costs about