To find uncertainties in different situations: The uncertainty in a reading: ± half the smallest division The uncertainty in a measurement: at least ±1 smallest division The uncertainty in repeated data: half the range i.e. ± ½ (largest - smallest value) The uncertainty in digital readings: ± the last significant digit unless otherwise quoted How to calculate absolute, fractional and.. Note: Relative error is undefined when the true value is zero.Also, relative error only makes sense when a measurement scale starts at a true zero.

Uncertainty Video Tutorial & Practice Channels for Pearson+

Question Video Finding the Uncertainty in a Given Quantity Squared Nagwa
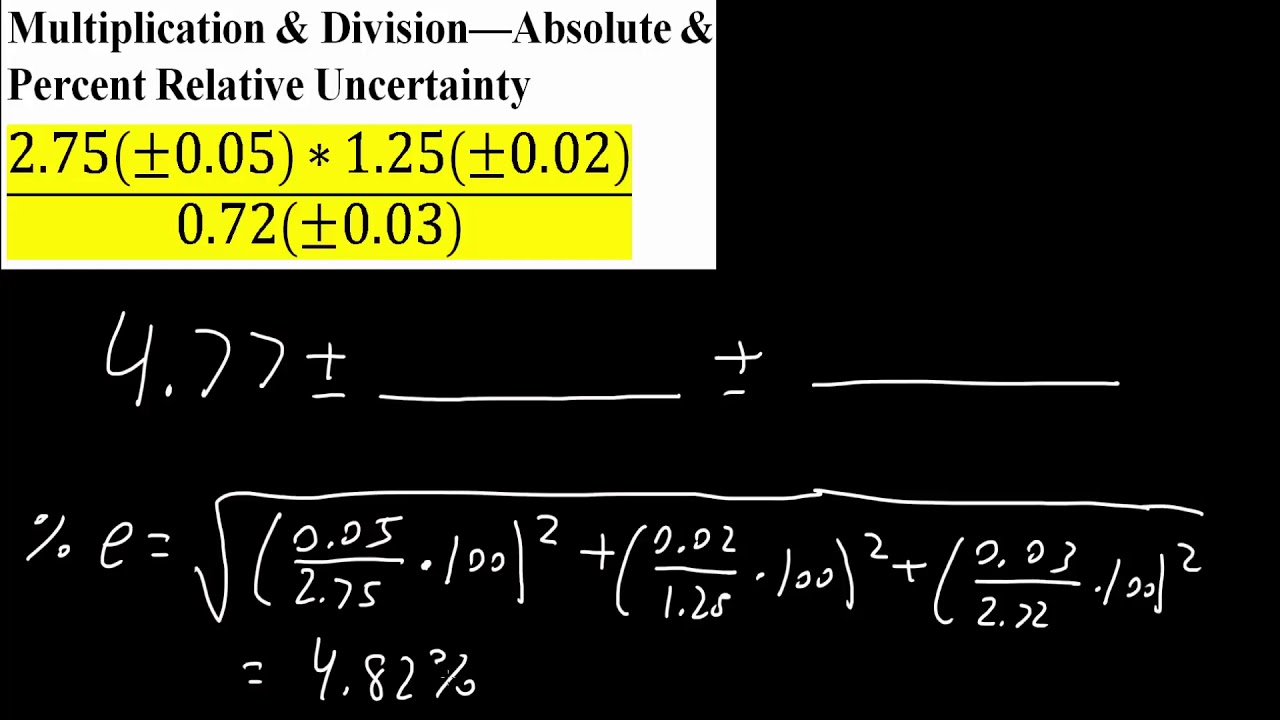
Multiplication & Division—Absolute & Percent Relative Uncertainty YouTube
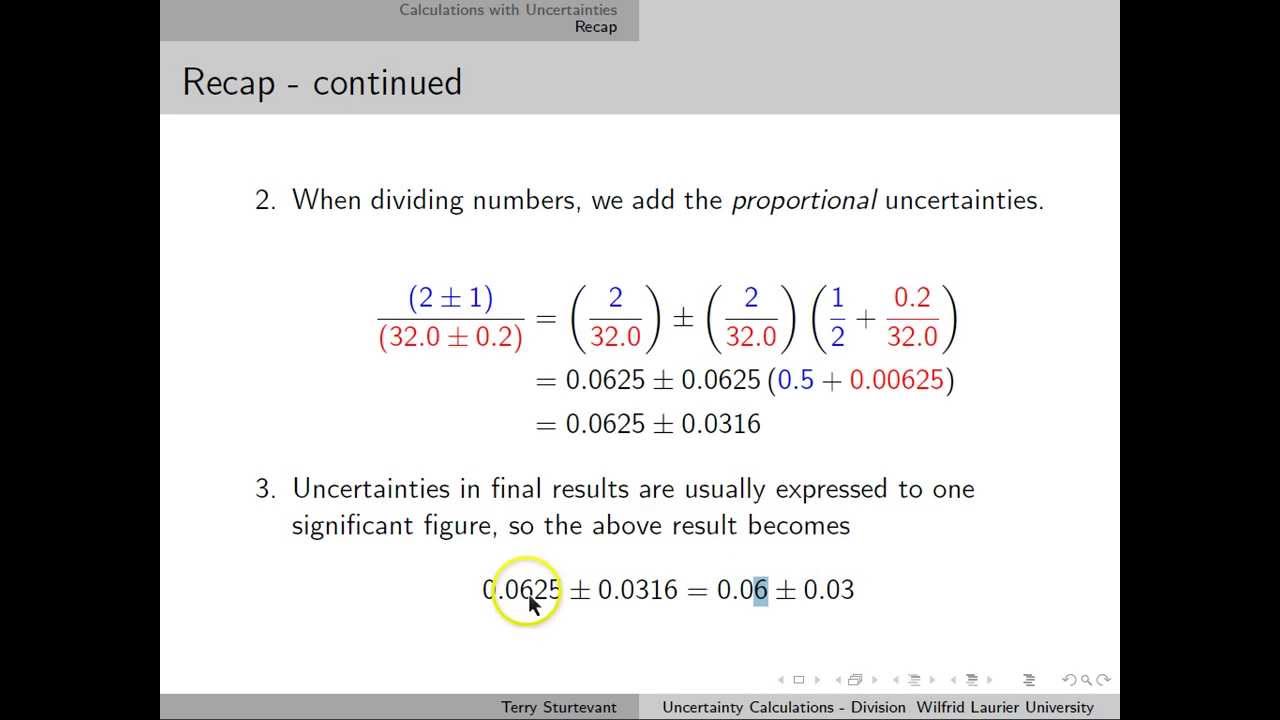
Uncertainty Calculations Division YouTube
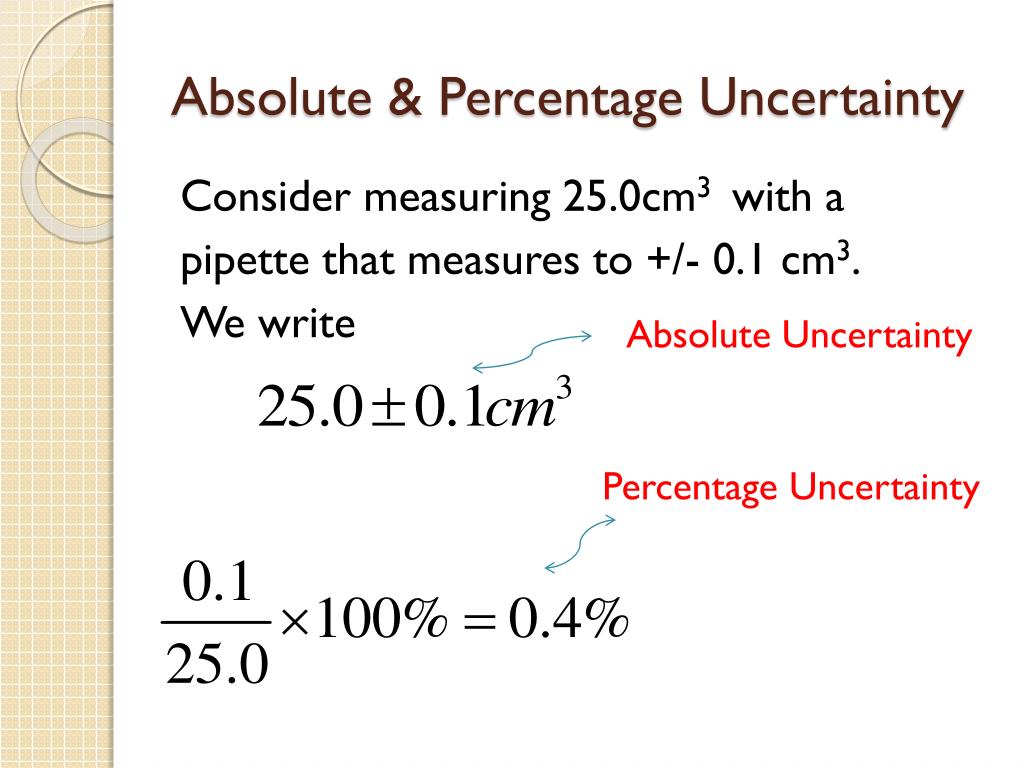
PPT Uncertainty & Errors in Measurement PowerPoint Presentation ID1901934
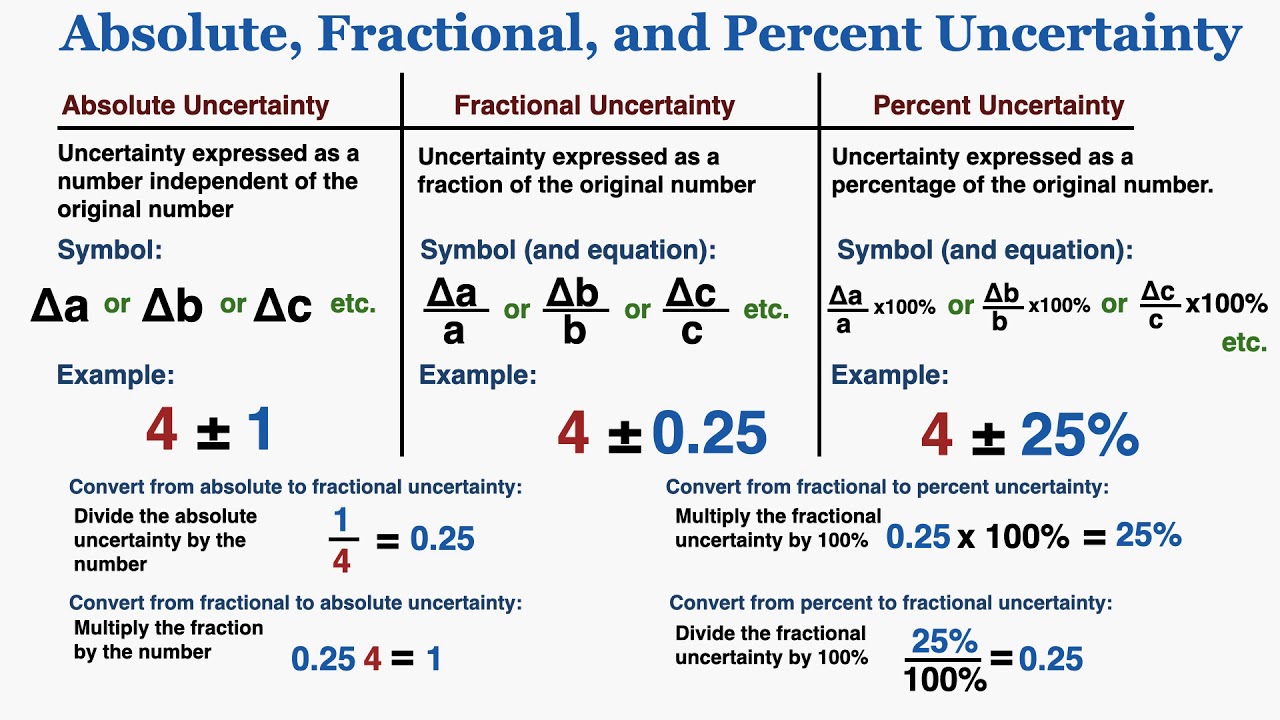
Absolute, Fractional, and Percent Uncertainty (With Examples) IB Physics YouTube
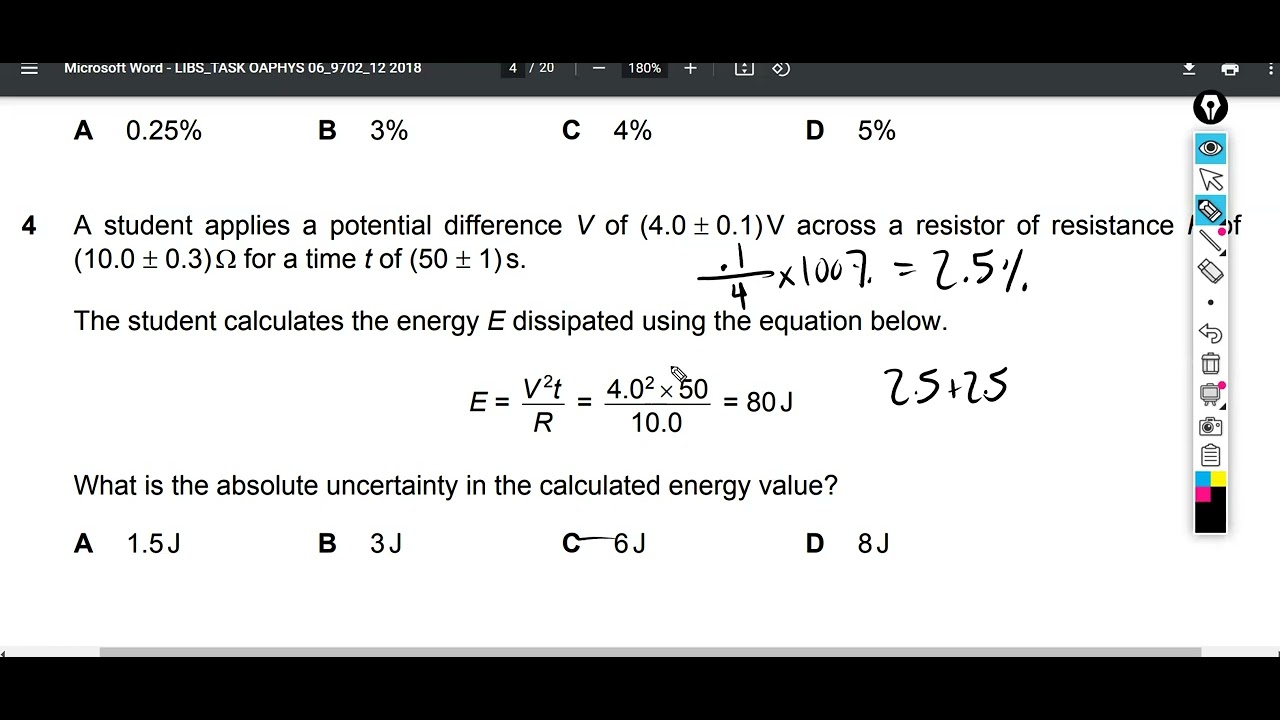
Calculating Absolute Uncertainty YouTube
[Solved] I NEED HELP WITH THE FOLLOWING QUESTIONS IN CHEMISTRY Perform the... Course Hero

Come Calcolare l'Errore Relativo 9 Passaggi

Absolute and Relative Error Part 1 Numerical Computation YouTube

Absolute Uncertainty vs Relative Uncertainty Analytical Chemistry YouTube

11.1 Absolute and percentage uncertainties YouTube

Calculating Uncertainties in Chemistry YouTube
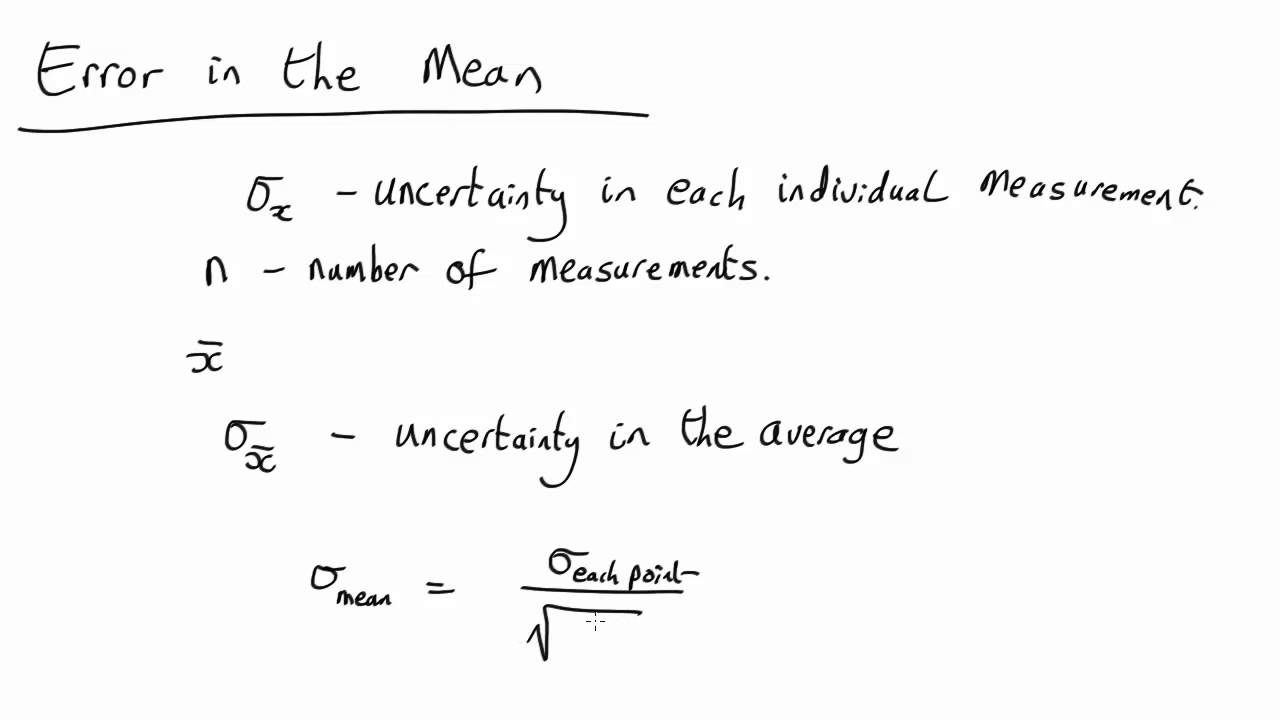
Uncertainty in mean YouTube

How To Calculate Absolute Error And Relative Error Tutorial Pics
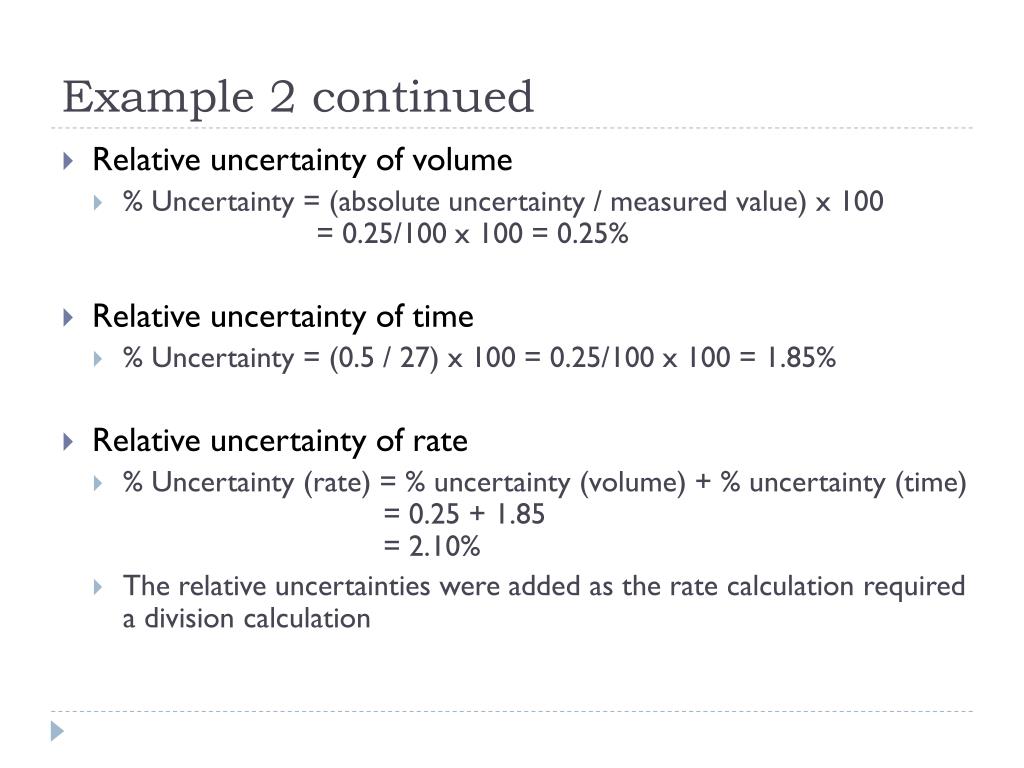
PPT Calculating Uncertainties PowerPoint Presentation, free download ID2474961
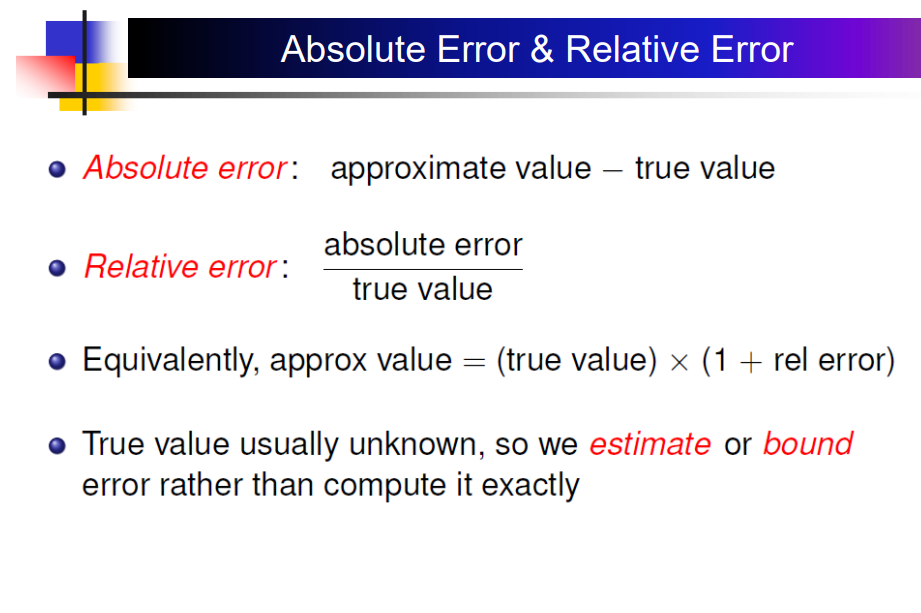
Solved Absolute Error & Relative Error Absolute error
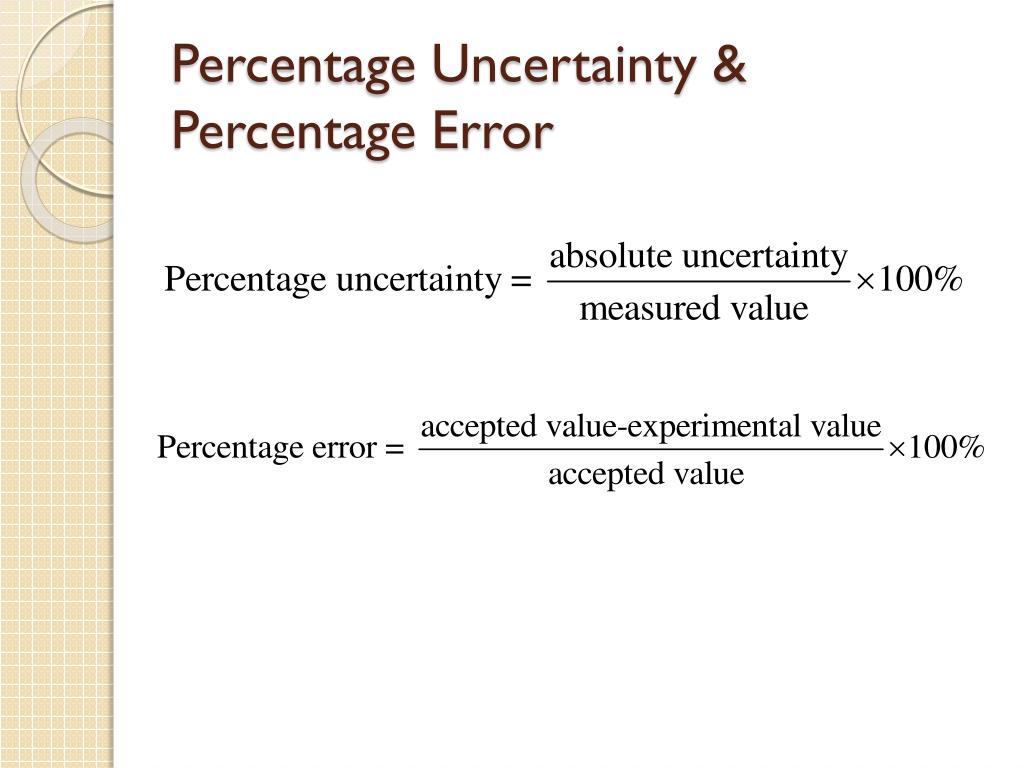
PPT Uncertainty & Errors in Measurement PowerPoint Presentation ID6303298
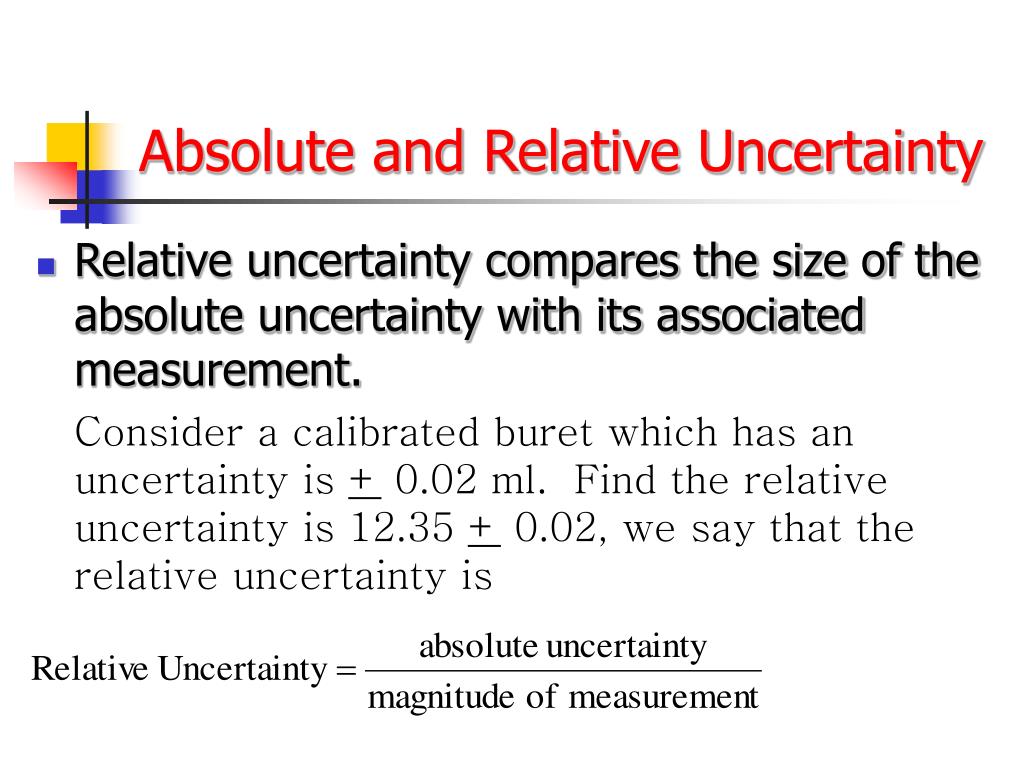
PPT Chapters 3 Uncertainty PowerPoint Presentation, free download ID5319211

Relative and Absolute Uncertainty in Dilution Calculations YouTube
Absolute Uncertainty Calculator. If you are human, leave this field blank. Welcome to the whimsical world of Absolute Uncertainty, where the numbers are made up and the precision matters (sort of)! Before you dive into this rollercoaster of digits and doubts, let's break the ice with a little introduction to our star of the show: the Absolute.. Quoting your uncertainty in the units of the original measurement - for example, 1.2 ± 0.1 g or 3.4 ± 0.2 cm - gives the "absolute" uncertainty. In other words, it explicitly tells you the amount by which the original measurement could be incorrect. The relative uncertainty gives the uncertainty as a percentage of the original value.